Abstract
Objective: This study aimed to evaluate the antiparasitic activity of Anvillea radiata plant extracts from the traditional pharmacopoeia by searching for new antiparasitic molecules. It seemed important to us to explore the nature of the natural antiparasitic substances in some Asteraceae of traditional Saharan medicine.
Methods: Our work focuses on the bioguided phytochemical study, identification and evaluation of the antiparasitic activity of the extract and pure compound of a medicinal species of the sahara Anvillea radiata (Asteracaea).
Results: The effect of plant extracts on parasites and the study of medicinal plants and endemic Saharan species Anvillea radiata by the use of different techniques of extraction, separation and purification were carried out on the aerial part of these plants tested against Giardia intestinalis. The objective of this study is to demonstrate the antiparasitic power of extracts from medicinal plants from the Bechar region. We estimated the inhibition of the parasites Giardia intestinalis subjected to extracts of medicinal plants. The compound exhibited a high anti-giardia activity, with growth inhibition of 96.24%.
Conclusion: These results suggest that Anvillea radiata has several biological activities and could provide a potential natural source of bioactive compounds and be beneficial to human health.
Introduction
Parasitic diseases caused by protozoa and helminths are a major health problem, killing millions every year while inflicting indelible injuries such as blindness or disfigurement on millions more. This will pave the way for innovative research to discover new bioactive molecules or bring to light classes of antiparasitic agents unknown until then. More than 60% of the medicines on the market are of natural origin, while only 10-20% of the world's flora have been studied for their phytochemical properties. This natural resource therefore remains very important1, 2, 3.
Giardiasis is one of the most common intestinal protozoan infections worldwide, affecting particular children4, 5. The causative agent, Giardia intestinalis, is a flagellated binucleated parasite that exists in two forms: infectious cysts and disease-causing trophozoites4. The main route of infection is through contaminated water, food or direct fecal–oral contact6. Symptoms of giardiasis include abdominal cramps and pain, nausea and diarrhea, which can lead to malabsorption and failure of children to thrive4.
The secondary metabolites, usually produced by plants for their defense mechanisms, are involved in the therapeutic properties of most medicinal plants7, 8. The search for new antiprotozoal drugs has become an urgent need. In this context, Anvillea radiata is a plant in the Asteraceae family that grows in northern Africa and particularly in Algeria. It is widely used in traditional medicine for the treatment of dysentery, gastric-intestinal disorders, chest cold, and hypoglycemic activity and has been reported to have antitumor activity9, 10, 11, 12.
During this study, we analyzed the anti-Giardia activity of Anvillea radiata fractionated by using chromatography. The purpose of the present study was to investigate the major compounds of Anvillea radiata extracts and to evaluate their biological properties, such as anti-giardia activity.
Methods
Vegetal Material
Anvillea radiata was collected from Bechar (southwest Algeria) in February and March 2018-2019. A voucher specimen has been deposited in the Phytochemistry and Organic Synthesis Laboratory (UTMB, Algeria) under accession N°CA 02/01.
Extraction and Isolation
Dried and powdered leaves (200 g) of Anvillea radiata were degreased with hexane and extracted with a mixture of water and methanol solution in a reflux apparatus. The extract solution was filtered and evaporated using a vacuum rotary evaporator. The resulting solution was extracted successively by extraction liquid/liquid with the solvents ethyl ether, ethyl acetate and n-butanol and separated by liquid chromatography on a column.
Antiparasitic test
The parasites used in this study Giardia intestinalis were obtained from the New Hospital in Bechar (Algeria).
Anti-Giardia assays
Giardia intestinalis was isolated in Dobell and Laidlaw Milieu and cultured in Diamond TYIS-33 pH 7.0 medium supplemented with 10% bovine serum and 0.5 mg/ml bovine bile at 37°C13. Trophozoites were cultured at 37°C in TYI-S-33 pH 7.8 medium supplemented with 5.0 mg/ml bovine bile14. Supplementation with 1 ml heat inactivated horse serum, oxacilline (1 g) and streptomycin sulfate (100 μg/ml). Giardia was incubated in TYI-S-33 with extracts at different concentrations: 0.5 mg/ml, 1 mg/ml, and 5 mg/ml for 6, 18 and 24 hours at 37°C. The percentage of growth inhibition of the parasite activity (%) was calculated according to the equation:
PI % = A control — A extract/A Control where
A: Absorbance of extract;
A control: Absorbance of temoin.
General procedure
UV spectra were obtained in MeOH with a Unicam UV 300 spectrophotometer and a Specord 200 Plus spectrophotometer. IR spectra were obtained with a Thermo Nicolet Avatar 320 FTIR spectrophotometer. The NMR spectra were taken on a Bruker Avance GP 250 (1H: 250 MHz; 13C: 63 MHz) spectrometer.
| N° | RMNC 13 | RMNH 1 | N° | RMNC 13 | RMNH 1 |
|---|---|---|---|---|---|
| 2 | 79 .63 | 5. 47, d, 1H | 2’ | 123.69 | 7. 39, d, 1H |
| 3 | 71.37 | 3.30, d ,1H | 3’ | 111.33 | 6. 64, d, 1H |
| 4 | 179.40 | - | 4’ | 146.57 | - |
| 5 | 174.90 | - | 5’ | 111.33 | 6.64, d, 1H |
| 6 | 94.45 | 6. 57, s, 1H | 6’ | 123.00 | 7. 39, d, 1H |
| 7 | 146.19 | - | 9 | 31.67 | 2. 18, 2.17, d, 2H |
| 8 | 111.03 | - | 10 | 34.13 | 1.68, m, 2H |
| 4a | 104.06 | - | 11 | 29.47 | 1. 63, m, 2H |
| 8a | 150.01 | - | 12 | 28.86 | 1. 61, m, 3H |
| 1’ | 131.97 | - | |||
Results and Discussion
The bioassay-guided fractionation of methanol extract led to the isolation of flavonoids, which were identified by spectroscopic techniques, as represented by the data below. UV max (MeOH): 293 nm, 301 nm, 347 nm. IR (cm–1) (KBr): 3317.3, 2825.3, 1965.2, 1444.3, 1108, 1019.4, 823.7, 762.2 cm-1.
The 1H and 13C NMR data of compound 1 are given in Table 1.
Examination of the RMN H1 spectrum showed a doublet at 3.30 ppm, which was attributable to H3 of a flavanone. The singlet at 6.57 ppm integrates 1H each, proton characteristics H-6, two other signals at 7.39 ppm attributable to H-2'and H-6' and the two signals at 6.64 ppm attributable to H-3'and H-5. The butane protons at 2.18 ppm, 1.68 ppm, 1.63 ppm and 1.61 ppm were attributed to H-9, H-10, H11, and H-12. Examination of the RMNC13 spectrum indicated the presence of the characteristic 3-carbon of a flavanone (71.37 ppm). We distinguished between aromatic CHs (at 123.69 and 111.33 ppm) and a carbonyl group at 179.4 ppm. The carbons of the butane part are C-9 (31.67), C-10 (34.13), C-ll (29.47), and C-12 (28.86)15, 16.
The above spectral data compared to the literature suggested that compound 1 is the 4’-méthoxyl, 3,7,5-tétrahydroxyl,8-butane flavanone (Figure 1).
Antiparasitic activity
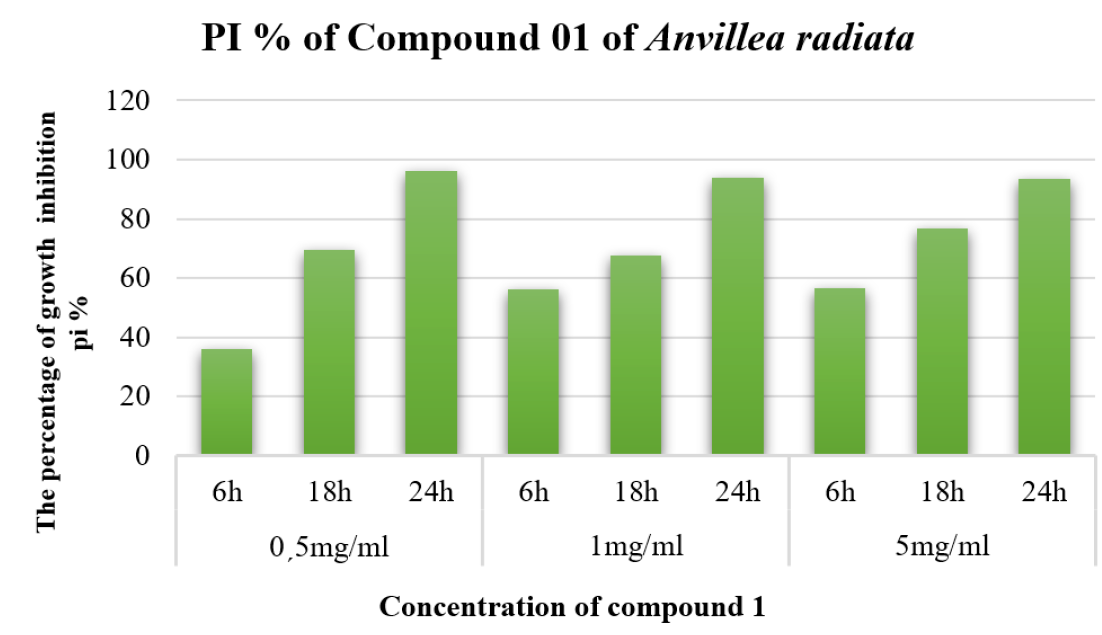
The results of the antiparasitic assay of the extract from the leaves of Anvillea radiata are presented in Figure 2. The extract was subjected to bioassay-guided fractionation to isolate the active compound responsible for Giardia intestinalis activity. Our studies demonstrate that compound 1, 4’-méthoxyl, 3,7,5-tétrahydroxyl, and 8-butane flavanone from Anvillea radiata extract also possess significant Giardia intestinalis growth inhibitory activity. The compound was tested against Giardia intestinalis, with a maximum PI of 96.24% (0.5 mg/ml). It seems interesting to us to point out that methanolic extracts are bioactive since they contain the majority of secondary plant metabolites. Therefore, Giardia intestinalis represents a major public health problem, although medicines exist to treat these diseases. These results were used to establish a search strategy for new therapeutic molecules for these plants. Anvillea radiata showed important inhibitory activity against Giardia intestinalis. By comparing the results, the findings show that the methanolic extract of the ethyl acetate fraction of Anvillea radiata is highly effective against Giardia intestinalis. Methanolic extracts were the most active in inhibiting the growth of Giardia intestinalis. The antiprotozoal activity of various extracts and fractions of Pulsatilla chinensis against Giardia intestinalis, including their effects on parasite growth, cell viability, adhesion and morphology17. Several studies have shown antiparasitaire activity against Trichomonas vaginalis and Giardia lamblia18, 19, 20, 21. Allium sativum extract has been tested for its anti-cardiac activity. Whole garlic extract gave a 24-hour IC50 of 0.3 mg/ml "122. By comparing our results, we found that the flavonoids of the separated plants were shown to have high efficacy against Giardia intestinalis compared to their results at lower concentrations and short times (0.5 mg/ml at 6 h).
Conclusion
The antiparasitic effect of the methanolic extract of Anvillea radiata at a dose of 5 mg/ml was evaluated in the present study, as part of the research by our laboratory on the biological activity and phytochemistry of medicinal plants in southwestern Algeria, we found it important to explore the nature of natural antiparasitic substances in some Asteraceae of traditional Saharan medicine.The chromatographic analysis of the ethyl acetate fraction of the water-methanol extract led to the isolation of flavonoid derivatives 4’-méthoxyl, 3,7,5-thiohydroxyl, and 8-butane flavanone for the first time.
Abbreviations
AR: Anvillea radiataGI: Giardia intestinalis
Acknowledgments
We acknowledged the director of Phytochemistry et Organic Synthesis Laboratory Pr. Dr. Abdelkrim Cheriti UTMB ,08000, Algeria.
Author’s contributions
All authors equally contributed to this work, read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Murray
C.J.,
Rosenfeld
L.C.,
Lim
S.S.,
Andrews
K.G.,
Foreman
K.J.,
Haring
D.,
Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet.
2012;
379
(9814)
:
413-31
.
View Article PubMed Google Scholar -
Touafek
O.,
Etude phytochimique de plantes médicinales du Nord et du Sud algériens. UNIVERSITE MENTOURICONSTANTINE, 2010. 2010
.
-
Thevenin
M.,
Développement de nouveaux agents antiparasitaires: vers la synthèse totale de la cissampeloflavone et de dérivés. 2013. Université Paris Sud-Paris XI. https://tel.archives-ouvertes.fr/tel-00871981. 2013
.
-
Wensaas
K.A.,
Langeland
N.,
Rortveit
G.,
Post-infectious gastrointestinal symptoms after acute Giardiasis. A 1-year follow-up in general practice. Family Practice.
2010;
27
(3)
:
255-9
.
View Article PubMed Google Scholar -
Feng
Y.,
Xiao
L.,
Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clinical Microbiology Reviews.
2011;
24
(1)
:
110-40
.
View Article PubMed Google Scholar -
Gardner
T.B.,
Hill
D.R.,
Treatment of giardiasis. Clinical Microbiology Reviews.
2001;
14
(1)
:
114-28
.
View Article PubMed Google Scholar -
Kumari
A.,
Sharma
R.,
A review on Millingtonia hortensis Linn. International Journal of Pharmaceutical Sciences Review and Research.
2013;
19
(2)
:
85-92
.
-
Znati
M.,
Ben Jannet
H.,
Cazaux
S.,
Souchard
J.P.,
Harzallah Skhiri
F.,
Bouajila
J.,
Antioxidant, 5-lipoxygenase inhibitory and cytotoxic activities of compounds isolated from the Ferula lutea flowers. Molecules (Basel, Switzerland).
2014;
19
(10)
:
16959-75
.
View Article PubMed Google Scholar -
Elagib
S.M.,
Antiparasitic activity of Eichhornia crassipes leaves extract. Biocatalysis and Agricultural Biotechnology.
2020;
101556
:
101556
.
View Article Google Scholar -
Bellakhdar
J.,
Pharmacopée marocaine traditionnelle. 1997: Ibis press. Available from: https://agris.fao.org/agris-search. 1997
.
-
Mebarki
L.,
Anvillea radiata as a source of natural antifungal compounds. African Journal of Pharmacy and Pharmacology.
2013;
7
(46)
:
2947-52
.
View Article Google Scholar -
Abdel Sattar
E.,
Galal
A.M.,
Mossa
G.S.,
Antitumor germacranolides from Anvillea garcinii. Journal of Natural Products.
1996;
59
(4)
:
403-5
.
View Article PubMed Google Scholar -
Keister
B.,
The relative energy variable in covariant nuclear wave functions. Nuclear Physics. A..
1983;
402
(3)
:
445-61
.
View Article Google Scholar -
Kane
A.V.,
Ward
H.D.,
Keusch
G.T.,
Pereira
M.E.,
In vitro encystation of Giardia lamblia: large-scale production of in vitro cysts and strain and clone differences in encystation efficiency. The Journal of Parasitology.
1991;
77
(6)
:
974-81
.
View Article PubMed Google Scholar -
Senatore
F.,
D'Agostino
M.,
Dini
I.,
Flavonoid glycosides of Barbarea vulgaris L. (Brassicaceae). Journal of Agricultural and Food Chemistry.
2000;
48
(7)
:
2659-62
.
View Article PubMed Google Scholar -
Berreghioua
A.,
Investigation phytochimique sur des extraits bioactifs de deux brassicaceae médicinales du sud Algerien: Moricandia arvensis et Zilla macroptera. diplôme de DoctoratUniversité Abou Balkaid -TLEMCEN 2016.
Google Scholar -
Li
L.D.,
Li
W.C.,
Liu
C.W.,
Shi
W.J.,
Gong
P.T.,
Li
J.H.,
Giardia intestinalis: effects of Pulsatilla chinensis extracts on trophozoites. Parasitology Research.
2012;
111
(5)
:
1929-35
.
View Article PubMed Google Scholar -
Zhu
F.,
Qin
C.,
Tao
L.,
Liu
X.,
Shi
Z.,
Ma
X.,
Clustered patterns of species origins of nature-derived drugs and clues for future bioprospecting. Proceedings of the National Academy of Sciences of the United States of America.
2011;
108
(31)
:
12943-8
.
View Article PubMed Google Scholar -
Pintong
A.R.,
Ruangsittichai
J.,
Ampawong
S.,
Thima
K.,
Sriwichai
P.,
Komalamisra
N.,
Efficacy of Ageratum conyzoides extracts against Giardia duodenalis trophozoites: an experimental study. BMC Complementary Medicine and Therapies.
2020;
20
(1)
:
63
.
View Article PubMed Google Scholar -
Mehriardestani
M.,
Aliahmadi
A.,
Toliat
T.,
Rahimi
R.,
Medicinal plants and their isolated compounds showing anti-Trichomonas vaginalis- activity. Biomedicine and Pharmacotherapy.
2017;
88
:
885-93
.
View Article PubMed Google Scholar -
Bero
J.,
Evaluation de l'activité antiparasitaire de plantes utilisées en médecine traditionnelle au Bénin et identification de principes actifs. UCL-Université Catholique de Louvain. 2012 . http://hdl.handle.net/2078.1/111492. 2012
.
-
Harris
J.C.,
Plummer
S.,
Turner
M.P.,
Lloyd
D.,
The microaerophilic flagellate Giardia intestinalis: allium sativum (garlic) is an effective antigiardial. Microbiology (Reading, England).
2000;
146
(Pt 12)
:
3119-27
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 8 No 3-4 (2022)
Page No.: Article ID789
Published on: 2022-11-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 2883 times
- PDF downloaded - 647 times
- XML downloaded - 78 times
 Biomedpress
Biomedpress



