Abstract
Diabetic foot ulcers (DFUs) present as a debilitating complication of diabetes which approximately affects 6.3% of the global population with significant mortality and morbidity consequences. While one-third of the total cost of diabetic care goes to DFU treatment, 20% patients have unresolved ulcers, while 40% experience recurrency within one year. To improve outcomes in DFU care, activated autologous platelet-rich plasma (aaPRP) is one of the adjuvant therapies proposed. Containing various bioactive proteins such as growth factors and cytokines, it possesses regenerative, antioxidative, anti-inflammatory, and antimicrobial qualities. Furthermore, its autologous nature, which also makes it a highly safe treatment modality, is also promising. In this review, we shortly discuss the pathogenesis of DFUs and how aaPRP may tackle the key players in DFU pathogenesis to improve wound healing outcomes. We also briefly describe its method of isolation and the current views on aaPRP from an authoritative source. Lastly, we summarize the existing evidence for the utilization of aaPRP in the treatment of DFUs.
Introduction
Diabetic foot ulcers (DFUs) present as a debilitating complication of diabetes which affects 6.3% of the global population. Some of the cited risk factors of DFUs include lower body mass index, longer diabetic duration, hypertension, and a history of smoking1. It is a severe public health issue which carries with it an immense burden of mortality and morbidity. Approximately 90,000 amputations are performed annually as a result of DFUs. This condition may also lead to other complications such as infection, abscesses, and pedal deformities2. The mortality rate associated with the development of DFUs is approximately 5% in 1 year, and 42% in 5 years3.
Given the current standard practices in the management of DFUs, it is estimated that DFU treatment takes up one-third of the total cost of diabetic care, which translated to US $176 billion in 2012. In spite of this high cost, around 20% of DFU patients have wounds that remain unhealed after one year. Besides resolution, recurrence is also an issue as about 40% of patients experience recurrency within one year3.
To improve outcomes in DFU care, several adjuvant therapies have been proposed, among which is activated autologous platelet-rich plasma (aaPRP). Advances in biotechnology has enabled scientists to utilize platelets for a wide array of uses, such as alopecia4, tendinopathy5, and even cytokine storm6, 7, 8. By processing blood samples from the patients, various bioactive proteins which possess favorable properties such as regeneration, can be released3, 9. This review aims to evaluate the current evidences regarding the efficacy of aaPRP in the treatment of DFUs.
Review
For this review, the author assembled credible literature from various databases such as PubMed, Scopus, and Wiley Online Library. The keywords used were “Diabetic Foot Ulcer”, “Platelet-Rich Plasma”, and “Platelet-Rich Plasma AND Diabetic Foot Ulcer”. Afterwards, the author handpicked relevant articles to be included in the review. Articles not written in English or that were deemed to be irrelevant were excluded.
In the end, a total of 50 studies were referenced in this review, of which eight are research articles focused on presenting current evidence of aaPRP in the treatment of DFUs. The other 42 are mainly review articles referenced in other parts of this article.
Pathogenesis of Diabetic Foot Ulcers
The pathogenesis of DFUs is complicated as it is a combination of several risk factors, such as peripheral neuropathy, peripheral vascular disease, pedal deformity, trauma, weakened resistance to infection, and more2. Here, some of the most dominant risk factors in the pathogenesis of DFU are discussed.
Diabetic Peripheral Neuropathy
Diabetic peripheral neuropathy (DPN) afflicts up to 66% and 59% of patients with type 1 and type 2 diabetes respectively10. The peripheral neurons supplying the feet are the longest cells in the body. As such, they require adequate vascularization, mitochondrial support, and glucose and lipid metabolism, all of which are unfortunately disturbed in diabetes11. As pointed out by experimental and clinical and studies, the most important factor in DPN pathogenesis is hyperglycemia-mediated cellular-injury12, 13. Other factors include obesity, dyslipidemia, impaired neurotrophic support, and microangiopathy which induce oxidative stress, mitochondrial dysfunction, and inflammation11.
One way that hyperglycemia causes DPN is through the polyol pathway, wherein excessive intracellular neuron glucose is converted into sorbitol, and into fructose afterwards by aldose reductase and sorbitol dehydrogenase, respectively. The ultimate increase in polyol flux causes intracellular hyperosmolarity and compensatory efflux of osmolytes, one of which is myoinositol, resulting in phosphatidylinositol depletion, hindering the creation of adenosine triphosphate. All of these processes result in Na+/K+ ATPase and protein kinase C dysfunction, impaired axonal transport, and neuronal breakdown. Furthermore, the reduction of glucose to sorbitol is also associated with NADPH consumption (which is required for the glutathione regeneration), thus contributing to oxidative stress14, 15.
In the presence of excess glucose, shunting to the hexosamine pathway also occurs. The conversion of fructose-6-phosphate to glucosamine-6-phosphate by glutamine: fructose-6-phosphate amidotransferase increases the production of uridine diphosphate N-acetylglucosamine that in turns modifies the transcription factor Sp1. This change results in altered gene expression and protein function. Consequently, vascular dysfunction, inflammation, and oxidative occurs11, 16.
Other than that, in a hyperglycemic environment, proteins, lipids, and nucleic acids can undergo irreversible non-enzymatic reactions which form advanced glycation products. These group of molecules, which are generated via the attachment of reactive carbohydrate groups, tend to affect cellular function negatively by disrupting the biological tasks of proteins. They also bind to their receptors which activates the intracellular signaling pathway, leading to inflammation, oxidative, stress, and nuclear degradation. This chain of events results in vascular dysfunction and deficits in neuronal conduction11, 14.
Peripheral Vascular Disease
Peripheral vascular disease (PVD) is an important risk factor in the development of DFU. PVD is responsible for about 50% of DFU cases and 70% of deaths in type 2 diabetes2. Hyperglycemia, dyslipidemia, and insulin resistance found in diabetics foster the development of PVD through mechanisms similar to those found in coronary artery disease17. The risk of PVD is higher and earlier in patients with diabetes due to endothelial dysfunction, vascular smooth muscle dysfunction, inflammation, and hypercoagulability. Other than diabetes, high blood pressure, smoking, dyslipidemia, and older age are also risk factors for PVD18. Diabetics, who are at a higher risk of atherosclerosis, may suffer from acute or chronic ischemia which may also lead to gangrene. Poor peripheral vascularization also leads to a poor wound healing process and further worsens the situation.2 Once it has become a neuroischemic ulcer, the wound is at risk of infection. While ischemia is not an independent risk factor for amputation, simultaneous occurrence with infection greatly increases the risk of limb amputation. Deep infections, such as osteomyelitis or soft tissue infection, are an immediate cause of amputation in 25 — 50% of DFU cases19.
Other Risk Factors
Other risk factors identified by studies include Charcot joints, a long duration of diabetes, retinopathy, nephropathy, hypertension, overweight, obesity, low socioeconomic level, foot deformity, and a history of previous DFUs and/or amputation20, 21, 22.
Current Views on Platelet-Rich Plasma in the Treatment of Diabetic Foot Ulcers
In 2016, the Wound Healing Society released guidelines regarding the treatment of DFUs23. The guidelines cover diagnosis, offloading, infection control, wound bed preparation, dressings, surgery, adjuvant agents, and the prevention of the recurrence of DFU. The evidence-based guidelines used well-controlled animal studies in addition to clinical studies. The strength of the evidence was then listed as Level I, Level II, or Level III with Level I evidence being the strongest due to being derived from meta-analyses or multiple laboratory studies supported by at least two clinical series.
Of interest to this review is the “Adjuvant Agents” section of the guidelines which cover topical, device, and systemic adjuvants for the treatment of DFUs. Level I evidence (very strong) was given to the application of platelet derived growth factor (PDGF)24, 25, 26, cytokines, and growth factors27, 28, 29 to improve DFU healing outcomes.
However, the guidelines stated that a systematic review of multiple trials studying the effects of aaPRP in the treatment DFU have not demonstrated improvements in the wound healing effects30. This finding is contradictory to the current literature and principles regarding the mechanism of aaPRP which will be discussed in a later part of this manuscript.
Isolation of Activated Autologous Platelet-Rich Plasma
There exists an abundance of aaPRP technique presented in the literatures but the end products are generally classified, as proposed by Ehrenfest et al.,31 as follows:
Current evidence shows that aaPRP preparations rich in leukocytes may impede healing by inducing inflammation through pro-inflammatory cytokines32.
One example of aaPRP isolation involves drawing whole blood from a patient through venipuncture into sodium citrate tubes, centrifuging the blood, separating the supernatant, and centrifuging it again at a faster speed. After this step, the lower-third of the tube will be considered platelet-rich plasma and the rest platelet-poor plasma, which would later be removed. The final product is a concentrated pellet of platelets suspended in plasma9.
Current Evidence for The Utilization of Autologous Platelet-Rich Plasma in Diabetic Foot Ulcers
Principles and Molecular mechanism
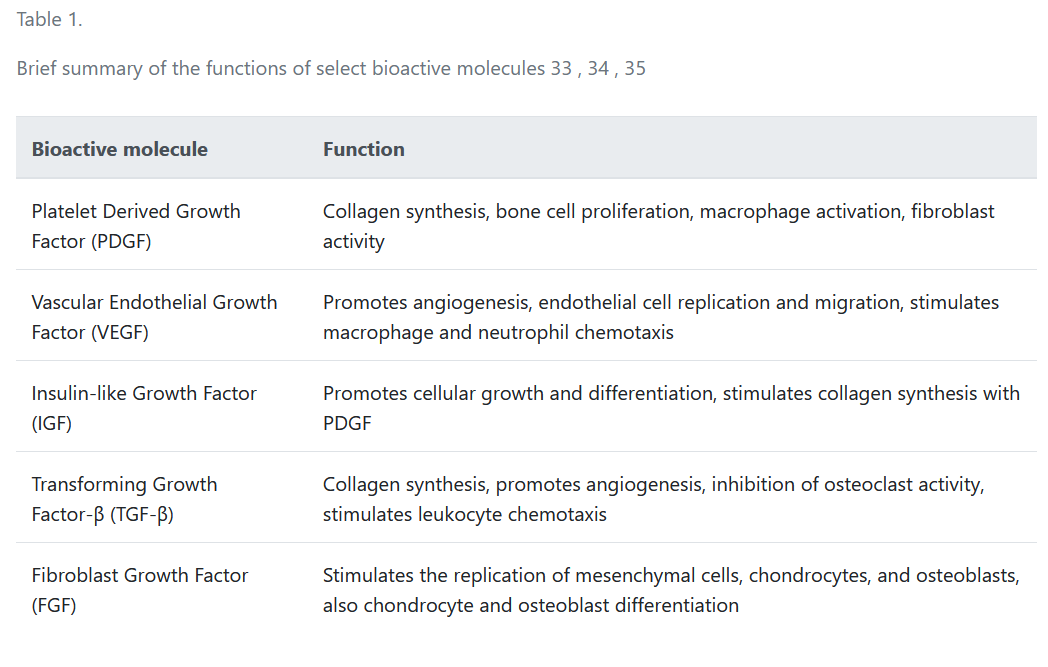
In DFUs, the lack of oxygen and nutrients hinders the epithelial cells from expressing growth factors, such as vascular endothelial growth factor (VEGF) and PDGF, which are vital for wound healing33, 34. With over 1500 bioactive factors found in platelets, aaPRP contains the much-needed growth factors which are beneficial for DFUs as the wounds are deficient in growth factors. Some examples of the growth factors include PDGF which has the ability to enhance collagen synthesis, fibroblast chemotaxis, and proliferation; VEGF which stimulates angiogenesis, the mitosis of endothelial cells, and increases vascular permeability; and the insulin-like growth factor (IGF) which promotes cell growth and differentiation, and also works with PDGF to stimulate collagen synthesis. Other growth factors such as transforming growth factor-β (TGF-β), epidermal growth factor (EGF), and fibroblast growth factor (FGF) are also present35. A summary of the regenerative functions of some of the bioactive molecules found in aaPRP are summarized in Table 1.
| Bioactive molecule | Function |
|---|---|
| Platelet Derived Growth Factor (PDGF) | Collagen synthesis, bone cell proliferation, macrophage activation, fibroblast activity |
| Vascular Endothelial Growth Factor (VEGF) | Promotes angiogenesis, endothelial cell replication and migration, stimulates macrophage and neutrophil chemotaxis |
| Insulin-like Growth Factor (IGF) | Promotes cellular growth and differentiation, stimulates collagen synthesis with PDGF |
| Transforming Growth Factor-β (TGF-β) | Collagen synthesis, promotes angiogenesis, inhibition of osteoclast activity, stimulates leukocyte chemotaxis |
| Fibroblast Growth Factor (FGF) | Stimulates the replication of mesenchymal cells, chondrocytes, and osteoblasts, also chondrocyte and osteoblast differentiation |
| Author (year) | Intervention | Control | Outcome |
|---|---|---|---|
| Parameter: Efficacy on Wound Healing | |||
| Driver, et al . (2006) 36 | aaPRP gel dressing | Saline gel dressing | Significantly higher healing rate and shorter time-to-heal in intervention |
| Ahmed, et al . (2017) 37 | aaPRP gel activated with thrombin | Antiseptic ointment gel | Significantly better healing rate and less infection in intervention group |
| Gupta, et al . (2021) 38 | aaPRP dressing and total-contact casting | Saline dressing and total-contact dressing | Intervention is no more efficacious than control in treating diabetic foot |
| Yarahmadi, et al . (2021) 39 | aaPRP fibrin glue dressing along with oral vitamin E and vitamin C | aaPRP fibrin glue dressing | Significant reduction of wound size in both groups. Significant decrease in inflammatory and oxidative markers in intervention group |
| Parameter: Safety and Accessibility | |||
| Karina, et al . (2021) 40 | Intravenous aaPRP | N/A | No observed adverse event (allergic reaction, infection, coagulation) |
| Linertová, et al . (2021) 41 | Usual care + manually obtained aaPRP vs. usual care + kit-obtained aaPRP vs. usual care | PRP for the management of DFU may be cost-effective or cost-saving if implemented appropriately | |
| Parameter: Others | |||
| Hassanien, et al . (2020) 42 | Perineural aaPRP injection | Medical treatment | Significant improvement in pain and numbness in DFU patients treated with intervention |
| Nolan, et al . (2020) 43 | aaPRP + fat graft vs. fat graft vs. usual care | Better fat graft survival and neovascularization in aaPRP + fat graft group. No clinical difference between the three groups. | |
Other than growth factors, a diverse array of cytokines are also present in platelets. These proteins can help in the modulation of inflammation and regeneration. Some of these include interleukin (IL)-1 receptor antagonist, IL-10, tumor necrosis factor-α, IL-12p70, and IL-844, 45. For example, IL-10 is an anti-inflammatory cytokine that is essential in wound healing as observed in models of fetal wound healing. The tissue repair observed is also not limited to cutaneous but also tendon and myocardial regeneration. Clinical trials have also demonstrated that wounds treated with IL-10 have less scarring46.
In the case of leukocyte-rich aaPRP, it is hypothesized that the neutrophils present in the preparation may impede wound healing by releasing massive amounts of reactive oxygen species and nitric oxide. While these secretions are useful to prevent microbial infection and to clear debris from the wound, the high number of leukocytes may delay wound healing. One possible way to control the amount of neutrophils in leukocyte-rich aaPRP is to increase the number of macrophages in the preparation as they induce neutrophil apoptosis. Nevertheless, leukocyte-rich aaPRP has its own uses and benefits outside the context of DFU healing35.
Safety and Accessibility
Accessibility-wise, the study carried out by Linertová, et al.41 in Spain deemed that aaPRP treatment for DFUs is a cost-saving alternative in most cases when obtained manually as opposed to commercial kits when compared to usual care.
aaPRP is highly safe for human administration. Being autologous in nature, aaPRP has virtually no chance for rejection, cross-contamination, or disease transmission. Safety-wise, there is no rejection and it is minimally invasive. Interestingly, Karina, et al.40 formulated an aaPRP preparation protocol that is also suitable for systemic administration via intravenous infusion. The study also proved that aaPRP prepared with such a technique is devoid of leukocytes and platelets after the last stage of activation. Additionally, the aaPRP in the study was also administered through a blood transfusion set to eliminate any potential cellular debris. Out of the more than 600 patients, none reported any adverse effects related to the systemic aaPRP administration.
Current Evidence and Study Results
A double-blind randomized controlled trial (RCT) in 2021 by Yarahmadi et al. found that aaPRP administration in a fibrin glue dressing (along with oral vitamin E and C supplementation) has the capability to help in DFU wound healing. This is evidenced by a significant wound size reduction and decrease in prooxidants compared to the control group39. This study results prove that aaPRP can decrease the immense oxidative stress brought about by hyperglycemia. On a side note, another RCT involving perineural aaPRP injection for DPN also showed an improvement in pain and numbness42. This encouraging result for improved peripheral nerve function may suggest that the benefits of aaPRP in DFU also extend beyond its antioxidative and anti-inflammatory properties.
Ahmed et al.37 also found that aaPRP in gel form is superior to a local antiseptic dressing in terms of healing rate and infection prevention in clean ulcers. In a previous canine study, aaPRP preparations have exhibited antimicrobial activity against methicillin-resistant Staphylococcus aureus47. This antimicrobial property may be attributed to the presence of a chemokine (C-C motif) ligand (CCL)-3, CCL5, and chemokine (C-X-C motif) ligand (CXCL)-1. Along with CCL3, CCL5, and CXCL1, transforming growth factor-β1 is also present and reported to be bacteriostatic. Furthermore, synergistic activity between aaPRP and antibiotics have also been observed48.
However, a recent RCT concluded that a PRP dressing is no more efficacious than a placebo of normal saline in DFU managed with total-contact casting38. The results of said study contradicts an older RCT done in 2006 by Driver et al.36 which found that 81.3% patients in the DFU group treated with aaPRP gel saw wound resolution compared to 42.1% patients in the saline gel group. The difference in administration and the presence of a total-contact cast may play a role in the difference of results; this is to be explored in future studies.
Studies combining aaPRP to enhance the regenerative potential and graft survival rates of mesenchymal stem cells also exist. The combination of aaPRP with stromal vascular fraction (SVF) may be implemented as a non-animal source of growth factors to support stem cell proliferation and differentiation49. A murine study of wound healing has also procured promising results, with the group of rats treated with SVF and aaPRP having the most accelerated wound healing compared to aaPRP or SVF alone50. An RCT further confirmed this dynamic between aaPRP and stem cells. Histological analyses demonstrated improved neovascularization and graft survival in DFU wounds, although these results are not accompanied by a shortened wound healing time43.
The findings of this literature review have been summarized in Table 2.
Conclusions
Along with the current principles of treatment, i.e., offloading, infection control, wound bed preparation, and others, aaPRP poses as an attractive adjunct treatment to DFU due to its efficacy, as well as its economic and safety profiles. As shown by its ability to improve wound closure, reduce oxidative stress, improve peripheral nerve function, prevent debilitating deep tissue infections, and overall improve safety, more clinicians should test aaPRP as an adjunct or alternative treatment for refractory DFUs. Future studies should explore the optimal form and dosage of aaPRP for DFU treatment.
Abbreviations
aaPRP: Activated autologous platelet-rich plasma
CCL: Chemokine (C-C motif) ligand
CXCL: Chemokine (C-X-C motif) ligand
DFU: Diabetic foot ulcer
DPN: Diabetic peripheral neuropathy
IL: Interleukin
PVD: Peripheral vascular disease
PDGF: Platelet derived growth factor
RCT: Randomized controlled trial
SVF: Stromal vascular fraction
VEGF: Vascular endothelial growth factor
Acknowledgments
None.
Author’s contributions
All authors equally contributed to this work, read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Zhang
P.,
Lu
J.,
Jing
Y.,
Tang
S.,
Zhu
D.,
Bi
Y.,
Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Annals of Medicine.
2017;
49
(2)
:
106-16
.
View Article PubMed Google Scholar -
Noor
S.,
Zubair
M.,
Ahmad
J.,
Diabetic foot ulcer: A review on pathophysiology, classification and microbial etiology. Diabetes & Metabolic Syndrome.
2015;
9
(3)
:
192-9
.
View Article PubMed Google Scholar -
Everett
E.,
Mathioudakis
N.,
Update on management of diabetic foot ulcers. Annals of the New York Academy of Sciences.
2018;
1411
(1)
:
153-65
.
View Article PubMed Google Scholar -
Khatu
S.S.,
More
Y.E.,
Gokhale
N.R.,
Chavhan
D.C.,
Bendsure
N.,
Platelet-rich plasma in androgenic alopecia: myth or an effective tool. Journal of Cutaneous and Aesthetic Surgery.
2014;
7
(2)
:
107-10
.
View Article PubMed Google Scholar -
Prodromos
C.C.,
Finkle
S.,
Prodromos
A.,
Chen
J.L.,
Schwartz
A.,
Wathen
L.,
Treatment of Rotator Cuff Tears with platelet rich plasma: a prospective study with 2 year follow-up. BMC Musculoskeletal Disorders.
2021;
22
(1)
:
499
.
View Article PubMed Google Scholar -
Karina
K.,
Rosliana
I.,
Rosadi
I.,
Sobariah
S.,
Christoffel
L.M.,
Novariani
R.,
Phase I/II Clinical Trial of Autologous Activated Platelet-Rich Plasma (aaPRP) in the Treatment of Severe Coronavirus Disease 2019 (COVID-19) Patients. International Journal of Inflammation.
2021;
2021
:
5531873
.
View Article PubMed Google Scholar -
Karina
K.,
Christoffel
L.M.,
Novariani
R.,
Rosadi
I.,
Rosliana
I.,
Rosidah
S.,
The Effect of Intravenous Autologous Activated Platelet-Rich Plasma Therapy on “Profibrotic Cytokine” IL-1β Levels in Severe and Critical COVID-19 Patients: A Preliminary Study. Sikavitsas VI, editor. Scientifica (Cairo) [Internet]. 2021;2021:1–7. 2021;
:
1-7
.
View Article Google Scholar -
Karina
K.,
Christoffel
L.M.,
Novariani
R.,
Rosadi
I.,
Rosliana
I.,
Rosidah
S.,
Case Report on Adjunct Intravenous Autologous Activated Platelet-Rich Plasma Therapy in Severely Ill COVID-19 Patients. Biomedical Research and Therapy.
2021;
8
(10)
:
4614-9
.
View Article Google Scholar -
Dhurat
R.,
Sukesh
M.,
Principles and methods of preparation of platelet-rich plasma: A review and author's perspective. Journal of Cutaneous and Aesthetic Surgery.
2014;
7
(4)
:
189-97
.
View Article PubMed Google Scholar -
Iqbal
Z.,
Azmi
S.,
Yadav
R.,
Ferdousi
M.,
Kumar
M.,
Cuthbertson
D.J.,
Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clinical Therapeutics.
2018;
40
(6)
:
828-49
.
View Article PubMed Google Scholar -
Sloan
G.,
Selvarajah
D.,
Tesfaye
S.,
Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nature Reviews. Endocrinology.
2021;
17
(7)
:
400-20
.
View Article PubMed Google Scholar -
Feldman
E.L.,
Callaghan
B.C.,
Pop-Busui
R.,
Zochodne
D.W.,
Wright
D.E.,
Bennett
D.L.,
Diabetic neuropathy. Nature Reviews. Disease Primers.
2019;
5
(1)
:
41
.
View Article PubMed Google Scholar -
Callaghan
B.C.,
Little
A.A.,
Feldman
E.L.,
Hughes
R.A.,
Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database of Systematic Reviews.
2012;
6
(6)
.
View Article PubMed Google Scholar -
Hosseini
A.,
Abdollahi
M.,
Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxidative Medicine and Cellular Longevity.
2013;
2013
:
168039
.
View Article PubMed Google Scholar -
Oates
P.J.,
Aldose reductase, still a compelling target for diabetic neuropathy. Current Drug Targets.
2008;
9
(1)
:
14-36
.
View Article PubMed Google Scholar -
Brownlee
M.,
Biochemistry and molecular cell biology of diabetic complications. Nature.
2001;
414
(6865)
:
813-20
.
View Article PubMed Google Scholar -
Thiruvoipati
T.,
Kielhorn
C.E.,
Armstrong
E.J.,
Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World Journal of Diabetes.
2015;
6
(7)
:
961-9
.
View Article PubMed Google Scholar -
Radha
T.P.,
P.S A, Annamalai S. Diabetes Mellitus and Peripheral Vascular Disease. Int J Contemp Med Res.
2020;
7
(7)
:
11-3
.
View Article Google Scholar -
Lepäntalo
M.,
Apelqvist
J.,
Setacci
C.,
Ricco
J.B.,
de Donato
G.,
Becker
F.,
Chapter V: diabetic foot. European Journal of Vascular and Endovascular Surgery.
2011;
42
:
60-74
.
View Article PubMed Google Scholar -
Al-Rubeaan
K.,
Al Derwish
M.,
Ouizi
S.,
Youssef
A.M.,
Subhani
S.N.,
Ibrahim
H.M.,
Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One.
2015;
10
(5)
:
e0124446
.
View Article PubMed Google Scholar -
Yazdanpanah
L.,
Shahbazian
H.,
Nazari
I.,
Arti
H.R.,
Ahmadi
F.,
Mohammadianinejad
S.E.,
Incidence and risk factors of diabetic foot ulcer: A population-based diabetic foot cohort (ADFC study)-two-year follow-up study. International Journal of Endocrinology.
2018;
2018
:
7631659
.
View Article PubMed Google Scholar -
Cardoso
H.C.,
Zara
A.L.,
Rosa
S.S.,
Rocha
G.A.,
Rocha
J.V.,
de Araújo
M.C.,
Risk Factors and Diagnosis of Diabetic Foot Ulceration in Users of the Brazilian Public Health System. Journal of Diabetes Research.
2019;
2019
:
5319892
.
View Article PubMed Google Scholar -
Lavery
L.A.,
Davis
K.E.,
Berriman
S.J.,
Braun
L.,
Nichols
A.,
Kim
P.J.,
WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair and Regeneration.
2016;
24
(1)
:
112-26
.
View Article PubMed Google Scholar -
Steed
D.L.,
Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plastic and Reconstructive Surgery.
2006;
117
(7)
:
143-9
.
View Article PubMed Google Scholar -
Bhansali
A.,
Venkatesh
S.,
Dutta
P.,
Dhillon
M.S.,
Das
S.,
Agrawal
A.,
Which is the better option: recombinant human PDGF-BB 0.01% gel or standard wound care, in diabetic neuropathic large plantar ulcers off-loaded by a customized contact cast?. Diabetes Research and Clinical Practice.
2009;
83
(1)
:
e13-6
.
View Article PubMed Google Scholar -
Blume
P.,
Driver
V.R.,
Tallis
A.J.,
Kirsner
R.S.,
Kroeker
R.,
Payne
W.G.,
Formulated collagen gel accelerates healing rate immediately after application in patients with diabetic neuropathic foot ulcers. Wound Repair and Regeneration.
2011;
19
(3)
:
302-8
.
View Article PubMed Google Scholar -
Hanft
J.R.,
Pollak
R.A.,
Barbul
A.,
van Gils
C.,
Kwon
P.S.,
Gray
S.M.,
Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. Journal of Wound Care.
2008;
17
(1)
:
30-2
.
View Article PubMed Google Scholar -
Uchi
H.,
Igarashi
A.,
Urabe
K.,
Koga
T.,
Nakayama
J.,
Kawamori
R.,
Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. European Journal of Dermatology.
2009;
19
(5)
:
461-8
.
View Article PubMed Google Scholar -
Cruciani
M.,
Lipsky
B.A.,
Mengoli
C.,
de Lalla
F.,
Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database of Systematic Reviews.
2013
.
View Article Google Scholar -
Martinez-Zapata
M.J.,
Martí-Carvajal
A.J.,
Solà
I.,
Expósito
J.A.,
Bolíbar
I.,
Rodríguez
L.,
Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database of Systematic Reviews.
2016
.
View Article Google Scholar -
Dohan Ehrenfest
D.M.,
Rasmusson
L.,
Albrektsson
T.,
Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends in Biotechnology.
2009;
27
(3)
:
158-67
.
View Article PubMed Google Scholar -
Dragoo
J.L.,
Braun
H.J.,
Durham
J.L.,
Ridley
B.A.,
Odegaard
J.I.,
Luong
R.,
Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. The American Journal of Sports Medicine.
2012;
40
(6)
:
1274-81
.
View Article PubMed Google Scholar -
Tran
T.D.,
Le
P.T.,
Van Pham
P.,
Diabetic foot ulcer treatment by activated platelet rich plasma: a clinical study. Biomedical Research and Therapy.
2014;
1
(2)
:
37-42
.
View Article Google Scholar -
Chen
Z.,
Fu
S.,
Wu
Z.,
Chen
J.,
Huang
Y.,
Wang
Y.,
Relationship between plasma angiogenic growth factors and diabetic foot ulcers. Clinica Chimica Acta.
2018;
482
:
95-100
.
View Article PubMed Google Scholar -
Pavlovic
V.,
Ciric
M.,
Jovanovic
V.,
Stojanovic
P.,
Platelet Rich Plasma: a short overview of certain bioactive components. Open Medicine (Warsaw).
2016;
11
(1)
:
242-7
.
View Article PubMed Google Scholar -
Nolan
G.S.,
Smith
O.J.,
Heavey
S.,
Jell
G.,
Mosahebi
A.,
Histological analysis of fat grafting with platelet-rich plasma for diabetic foot ulcers-A randomised controlled trial. International Wound Journal.
2022;
19
(2)
:
389-98
.
View Article PubMed Google Scholar -
Hassanien
M.,
Elawamy
A.,
Kamel
E.Z.,
Khalifa
W.A.,
Abolfadl
G.M.,
Roushdy
A.S.,
Perineural platelet-rich plasma for diabetic neuropathic pain, could it make a difference?. Pain Medicine.
2020;
21
(4)
:
757-65
.
View Article PubMed Google Scholar -
Linertová
R.,
Del Pino-Sedeño
T.,
Pérez
L.G.,
Aragón-Sánchez
J.,
Andia-Ortiz
I.,
Trujillo-Martín
M.,
Cost-effectiveness of Platelet-Rich Plasma for Diabetic Foot Ulcer in Spain. The International Journal of Lower Extremity Wounds.
2021;
20
(2)
:
119-27
.
View Article PubMed Google Scholar -
Karina
K.,
Ekaputri
K.,
Biben
J.A.,
Purwoko
R.H.,
Sibuea
T.P.,
Astuti
S.L.,
Evaluating the Safety of Intravenous Delivery of Autologous Activated Platelet-rich Plasma. Journal of Health Sciences (Sarajevo).
2021;
11
(2)
:
61-5
.
View Article Google Scholar -
Yarahmadi
A.,
Saeed Modaghegh
M.H.,
Mostafavi-Pour
Z.,
Azarpira
N.,
Mousavian
A.,
Bonakdaran
S.,
The effect of platelet-rich plasma-fibrin glue dressing in combination with oral vitamin E and C for treatment of non-healing diabetic foot ulcers: a randomized, double-blind, parallel-group, clinical trial. Expert Opinion on Biological Therapy.
2021;
21
(5)
:
687-96
.
View Article PubMed Google Scholar -
Gupta
A.,
Channaveera
C.,
Sethi
S.,
Ranga
S.,
Anand
V.,
Efficacy of intralesional platelet-rich plasma in diabetic foot ulcer. J Am Podiatr Med Assoc.
2021;
111
(3)
:
Article_7
.
View Article Google Scholar -
Ahmed
M.,
Reffat
S.A.,
Hassan
A.,
Eskander
F.,
Platelet-Rich Plasma for the Treatment of Clean Diabetic Foot Ulcers. Annals of Vascular Surgery.
2017;
38
:
206-11
.
View Article PubMed Google Scholar -
Driver
V.R.,
Hanft
J.,
Fylling
C.P.,
Beriou
J.M.,
Autologel Diabetic Foot Ulcer Study Group
A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy/Wound Management.
2006;
52
(6)
:
68-70
.
PubMed Google Scholar -
Cassano
J.M.,
Kennedy
J.G.,
Ross
K.A.,
Fraser
E.J.,
Goodale
M.B.,
Fortier
L.A.,
Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surgery, Sports Traumatology, Arthroscopy.
2018;
26
(1)
:
333-42
.
View Article PubMed Google Scholar -
Pochini
A.C.,
Antonioli
E.,
Bucci
D.Z.,
Sardinha
L.R.,
Andreoli
C.V.,
Ferretti
M.,
Analysis of cytokine profile and growth factors in platelet-rich plasma obtained by open systems and commercial columns. Einstein (Sao Paulo, Brazil).
2016;
14
(3)
:
391-7
.
View Article PubMed Google Scholar -
King
A.,
Balaji
S.,
Le
L.D.,
Crombleholme
T.M.,
Keswani
S.G.,
Regenerative Wound Healing: The Role of Interleukin-10. Advances in Wound Care (New Rochelle, N.Y.).
2014;
3
(4)
:
315-23
.
View Article PubMed Google Scholar -
Farghali
H.A.,
AbdElKader
N.A.,
AbuBakr
H.O.,
Aljuaydi
S.H.,
Khattab
M.S.,
Elhelw
R.,
Antimicrobial action of autologous platelet-rich plasma on MRSA-infected skin wounds in dogs. Scientific Reports.
2019;
9
(1)
:
12722
.
View Article PubMed Google Scholar -
Zhang
W.,
Guo
Y.,
Kuss
M.,
Shi
W.,
Aldrich
A.L.,
Untrauer
J.,
Platelet-rich plasma for the treatment of tissue infection: preparation and clinical evaluation. Tissue Engineering. Part B, Reviews.
2019;
25
(3)
:
225-36
.
View Article PubMed Google Scholar -
Karina
K.,
Rosliana
I.,
Rosadi
I.,
Schwartz
R.,
Sobariah
S.,
Afini
I.,
Safety of technique and procedure of stromal vascular fraction therapy: from liposuction to cell administration. Scientifica.
2020;
2020
:
2863624
.
View Article PubMed Google Scholar -
Karina
K.,
Samudra
M.F.,
Rosadi
I.,
Afini
I.,
Widyastuti
T.,
Sobariah
S.,
Combination of the stromal vascular fraction and platelet-rich plasma accelerates the wound healing process: pre-clinical study in a Sprague-Dawley rat model. Stem Cell Investigation.
2019;
6
(18)
:
18
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 8 No 1 (2022)
Page No.: Article ID 788
Published on: 2022-06-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 3307 times
- PDF downloaded - 861 times
- XML downloaded - 156 times
 Biomedpress
Biomedpress

